LOCAL ANESTHETICS
- Local anesthetics are drugs which upon topical application or local injection cause reversible loss of sensory perception, especially of pain in a localized area of the body.
- It blocks generation and conduction of nerve impulses at a localized site of contact without structural damage to neurons.
- Loss of sensory as well as motor impulses.
- LAs, do not cause the loss of consciousness when administered correctly.
HISTORY OF LOCAL ANESTHETICS
- Albert Niemann (1860) isolated crystals from the coca shrub – and called it “COCAINE”, he found that it reversibly numbed his tongue.
- German chemist Alfred Einhorn (1905) produced the first synthetic ester type local anesthetic Novocain (procaine) which retained the nerve blocking properties but lacked the powerful CNS actions of cocaine.
- Swedish chemist Nils Lofgren (1943) synthesized the first amide-type local anesthetic – marketed under the name of Xylocaine (Lidocaine).
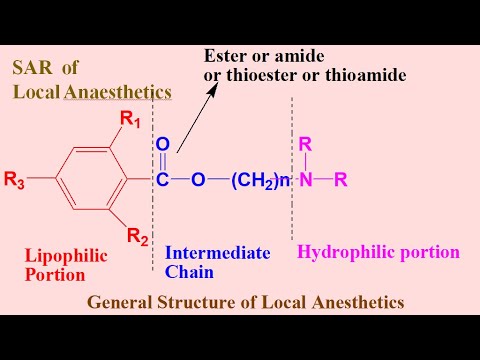
CHEMISTRY OF LOCAL ANESTHETICS
- Three major parts of any Local Anesthetics:
- Aromatic Ring (Lipophilic Moiety).
- Intermediate Chain is of Amide or Ester (basis of classification).
- Amine Group (Hydrophilic Group).
- Potency = Lipid Solubility
- Higher solubility = Can use a lower concentration and reduce potential for toxicity.
CLASSIFICATION OF LOCAL ANESTHETICS
1.Based on duration of action
Duration = Protein Binding
- Short Acting (20-25 Min.):
- Procaine
- Chloroprocaine.
- Intermediate Acting (45-60 Min.):
- Lidocaine (Lignocaine)
- Prilocaine
- Lignocaine
- Cocaine
- Long Acting (2-3 Hours):
- Bupivacaine
- Etidocaine
- Ropivacaine
- Tetracaine
2. Based on chemical nature:
1.Esters:
- Cocaine
- Procaine
- Tetracaine
- Benzocaine
- Produce more intense & longer lasting anesthesia.
- Not hydrolyzed by plasma esterase’s.
- Rarely cause hypersensitivity reactions.
2.Amides:
- Lidocaine
- Mepivacaine
- Bupivacaine
- Etidocaine
- Short duration of action and less analgesia.
- Hydrolyzed by plasma esterase’s.
- High risk of hypersensitivity.
PROPERTIES OF LOCAL ANESTHETICS
- Reversible in action
- Nonirritant
- No allergic reaction
- No systemic toxicity
- Rapid onset of action
- Sufficient duration of action
- Potent
- Stable in solution
- No interfere with healing of tissues
- Should have Vaso-constrictive action
- Not expensive
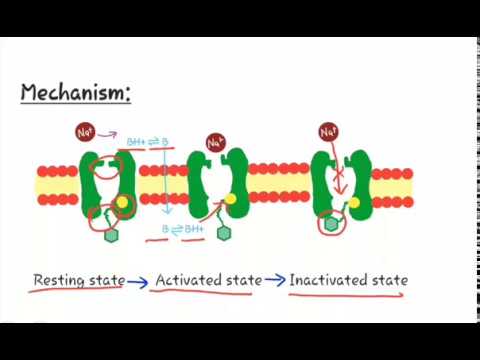
MECHANISM OF ACTION
- The primary target of the LA, Voltage Activated Sodium Channels (VASA) is one the numerous membrane proteins which reside in phospholipids bilayer encapsulating the neurons.
- LAs block VASA → Reduce Na+ influx → No depolarization → No Conduction of Active Potential (PA).
- Local anesthetics gain access to the inner axonal membrane by:
- Traversing sodium channels while they are more often in an open configuration.
- Pass directly through the plasma membrane.
- More lipid soluble (Unionized/Uncharged) form → More effective intracellular conc.
- Inside the neuron → Ionized form is more effective blocking entity.
- Both ionized & unionized forms play significant role in
- First in reaching the receptor site.
- Second in causing the effect.
PHARMACOKINETICS OF LOCAL ANESTHETICS
- ABSORPTION:
- Absorption is determined by:
- Absorption site
- Dose
- Rate of injection
- Pharmacological properties.
- Plasma Conc. After injection at various sites is:
- Intrapleural > Intercostal > Lumbar Epidural > Brachial Plexus > Sciatic > Femoral.
- First pass Pulmonary Metabolism limits the Conc. Of Local Anesthetics that reaches to Systemic Circulation.
- Absorption is determined by:
FACTORS AFFECTING THE ABSORPTION OF LAs:
- Site of Injection (Intrapleural > Intercostal > Lumbar Epidural > Brachial Plexus > Sciatic > Femoral).
- Dose
- Physicochemical Properties (Lipid Solubility & Protein Binding).
- Addition of Epinephrine.
2.DISTRIBUTION:
- Tissue distribution of LA is proportional to:
- Lipid solubility of drug
- Blood supply to that tissue
- LA drugs are distributed rapidly in:
- Brain, Heart, Liver, Lung.
- But more slowly distributed…. which have lower blood supply:
- Muscles & Adipose Tissues.
- Patient age, cardio-vascular status and hepatic function influence the tissue blood flow.
3.METABOLISM:
- Amide:
- Metabolism is dependent on hepatic blood flow.
- Toxicity of amides is more likely with:
- Prolonged infusions in sick, elderly patients.
- Postoperative increase in AAG (Acute Angle Glaucoma) attenuates the rise in plasma concentrations
- Esters:
- Hydrolyzed rapidly in plasma by pseudo cholinesterase to the metabolite
- Para-aminobenzoic acid (PABA), which can generate an allergic reaction.
4.EXCRETION:
- Kidneys are primary excretory organs of LA drugs & their metabolites.
- Esters appears in very small conc. of parent compound in urine.
- Excretion of amide local anesthetics is dependent on hepatic metabolism metabolites may accumulate in renal failure.
- Metabolism is fastest in the rank order:
- Prilocaine > Lidocaine > Bupivacaine.
- Most of the LA drugs cross the placenta.
IMPORTANCE OF ADDING VASOCONSTICTORS TO LOCAL ANESTHETICS
- Vasoconstrictors Constrict blood vessels
- Decrease blood flow
- Decrease the blood level of the drug
- Increase the concentration of drug at the site
- Decrease bleeding at site
- Increase the duration of drug.
