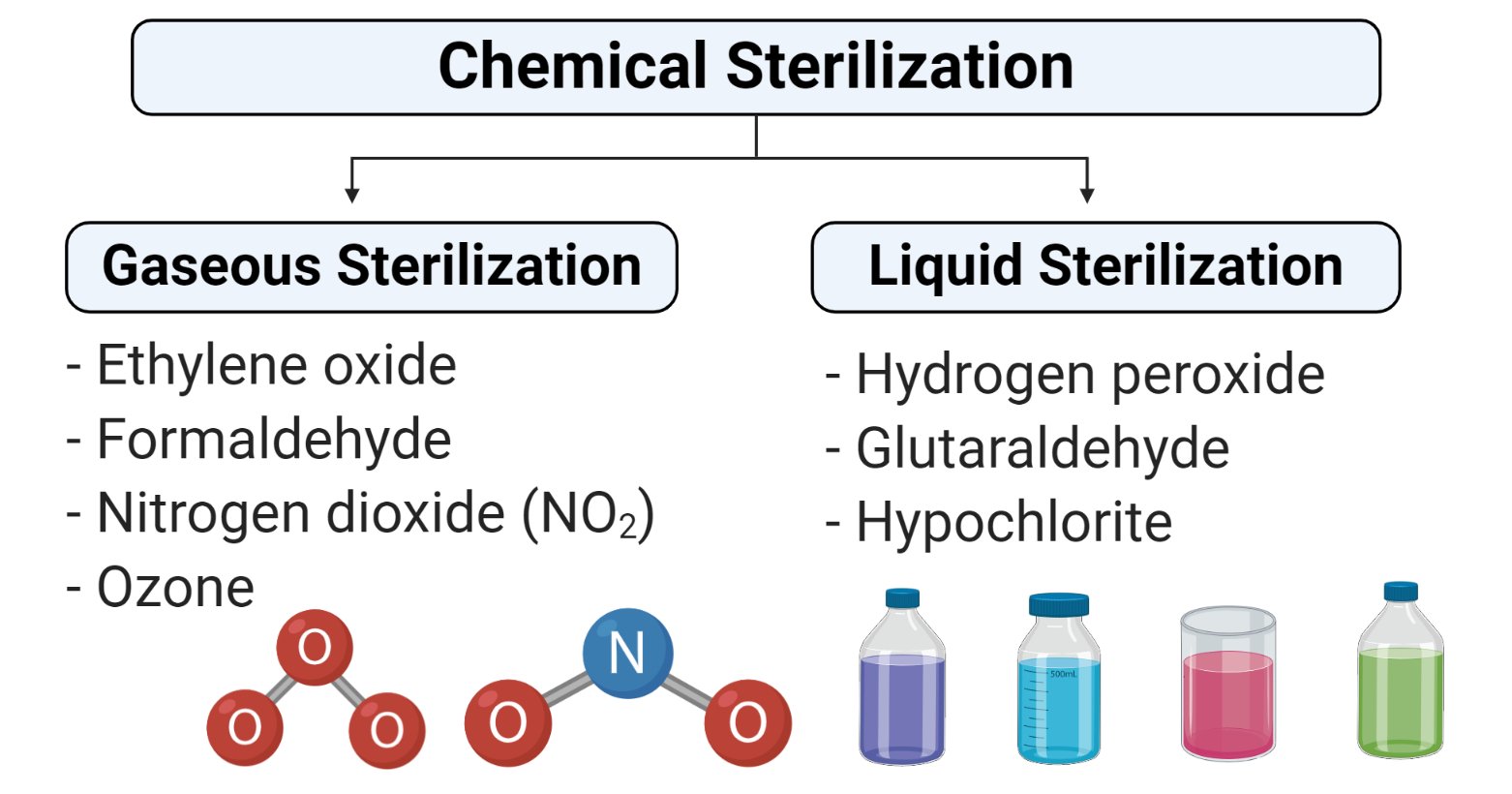
Chemical sterilization involves the utilization of certain chemicals to cause microbial termination.
For this, the most used materials are chlorine, formaldehyde, glutaraldehyde, quaternary ammonium salts, and ETO.
Alkylating gases
- Principle: Alkalization of essential metabolites of microbes, affecting reproductive
Alkalization occurs by replacing hydrogen on sulfhydryl, amino, carboxyl, hydroxyl-groups with a hydroxyl radical
- Example: ethyleneoxide,betapropiolactone,glutaraldehyde,formaldehyde,propylene oxide
Ethylene oxide
- Cyclic ether, reactivity is because of oxygen bridge
- Gas at room temperature
- Alone, highly flammable, when mixed with air explosives’ admixed with CO2 or Freon’s.
- High penetrability through plastic, paperboard, powder
- Chemically inert towards most solid materials
- Working conditions: conc.800–1200mg/L, with operating temperature 45–63°C ,humidity>30%
Equipment design and operation
- An ethylene oxide sterilizer consists of a leak-proof and explosion-proof steel chamber, normally of 100–300-litre capacity, which can be surrounded by a hot-water jacket to provide a uniform chamber temperature
- Successful operation of the sterilizer requires removal of air from the chamber by evacuation, humidification and conditioning of the load by passage of sub atmospheric pressure steam followed by a further evacuation period and the admission of preheated vaporized ethylene oxide from external pressurized canisters or single-charge cartridges.
Sterilization process
- Material is placed in a room or chamber and exposed to RH of upto 98% for 60 mints or more.
- It is then placed in the chamber,previously heated to about 55 centigrades,and an intial vaccum of approximately 27 in,Hg is drawn.The EtO is then introduced along with moisture,to achieve a relative humidity of 50 to 60%,to pressure required to give desired conc,of gas,which is maintained.Following exposure period of 6 to 24 hrs,gas is exhausted,vaccum of approx.25 in.Hg is drawn.
- Filtered air is introduced untill atomoshpheric pressure is attained.
Factors affecting sterilization process
1.Heat
A rise in temperature of 17 degree permits shortening of exposure period by one half.
2.Humidity
30% or more is essential for antimicrobial effect
3.Concentration of gas
Applications
- Dry powders
- Plastic materials, paperboard, rubber materials
- Optical instruments
- Parenteral administration sets
- Hypodermic needles
- Plastic syringe
Advantages:
- High penetrability
- Chemically inert
- Use of low temperature
- low exposure period
- Easy to dissipate
Disadvantages:
- Tissue irritation
- Carcinogenic mutagenic
Limitation
- Alkalization may also occur of drug substances particularly in liquid form,so limited essentially to dry powders
Other alkylating gases
Beta-propiolactone: concentration 2 to 4 mg/l, temp.>24 Celsius, humidity at least 70%, exposure period at least 2 hours, poor penetrability so for sterilization of surfaces in large spaces
Glutaraldehyde: in solution form, for surgical instruments LTSF
Oxidizing Gases
Oxidizing agents act by oxidizing the cell membrane of microorganisms, which results in a loss of structure and leads to cell lysis and death.
- Hydrogen Peroxide
- Ozone
- Chlorine Dioxide
- Peracetic acid
Hydrogen Peroxide
- Effective against spores at a range of temperature.
- Hydrogen peroxide is used to sterilize heat or temperature-sensitive articles.
- Greatest action when used at near-saturation levels on clean dry surface.
- Does not leave a toxic residue.
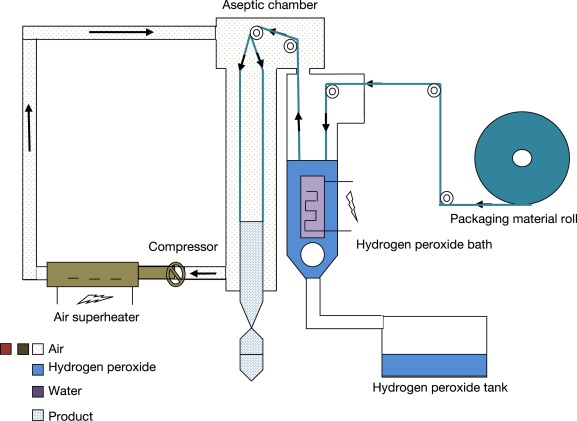
- Vapor is usually obtained by evaporation of heated stock solution.
- The biggest advantage of hydrogen peroxide as a sterilant is the short cycle time. Whereas the cycle time for ethylene oxide may be 10 to 15 hours.
Disadvantages
- Drawbacks of hydrogen peroxide include material compatibility, a lower capability for penetration and operator health risks.
- Products containing cellulose, such as paper, cannot be sterilized using VHP and products containing nylon may become brittle.
- The penetrating ability of hydrogen peroxide is not as good as ethylene oxide and so there are limitations on the length and diameter of the lumen of objects that can be effectively sterilized.
- Hydrogen peroxide is a primary irritant and the contact of the liquid solution with skin will cause ulceration depending on the concentration and contact time.
- The vapour is also hazardous, primarily affecting the eyes and respiratory system.
Ozone
- It is used in industrial settings to sterilize water and air, as well as a disinfectant for surfaces.
- Ozone is a very efficient sterilant because of its strong oxidizing properties capable of destroying a wide range of pathogens including prions.
- The disadvantage of using ozone is that the gas is very reactive and very hazardous
Chlorine Dioxide
- It has sporicidal activity.
- Currently under commercial development.
- Applications are limited due to its effect on materials (uncoated Al foil, copper, polycarbonate and polyurethane.
Peracetic Acid
- 0.2% is a recognized sterilant by the FDA.
- Vapourized from is used to sterilize isolators.
- Long contact time is needed though.
- Can cause corrosion of metals and rubber.
- Low penetrating power.