Ultraviolet (UV) spectroscopy is commonly used to analyze organic compounds, including various drugs. UV spectroscopy measures the absorption of ultraviolet light by molecules, providing information about their electronic structure. Many drugs exhibit characteristic absorption patterns in the UV range, allowing for their identification and quantification. Here are some classes of drugs that are often analyzed using UV spectroscopy:
- Analgesics and anti-inflammatory drugs: Examples include aspirin, ibuprofen, acetaminophen (paracetamol), and indomethacin.
- Antibiotics: Drugs like penicillin, tetracycline, erythromycin, and ciprofloxacin can be analyzed using UV spectroscopy.
- Antidepressants: Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine, sertraline, and escitalopram are often studied with UV spectroscopy.
- Antihistamines: Drugs like diphenhydramine, loratadine, and cetirizine can be analyzed using UV spectroscopy.
- Antipsychotics: Medications such as haloperidol, risperidone, and olanzapine are commonly studied using UV spectroscopy.
- Antiviral drugs: Examples include acyclovir, lamivudine, and zidovudine, which can be analyzed using UV spectroscopy.
- Cardiovascular drugs: Drugs like nitroglycerin, atenolol, and verapamil can be analyzed using UV spectroscopy.
- Hypnotics and sedatives: Medications such as zolpidem, diazepam, and phenobarbital can be studied using UV spectroscopy.
- Nonsteroidal anti-inflammatory drugs (NSAIDs): Besides analgesics, drugs like naproxen, diclofenac, and piroxicam fall into this category and can be analyzed using UV spectroscopy.
UV spectroscopy is a useful tool for drug analysis, it may not be sufficient for comprehensive identification and quantification. Other techniques such as high-performance liquid chromatography (HPLC) or mass spectrometry (MS) are often used in conjunction with UV spectroscopy to enhance the analysis of drugs.
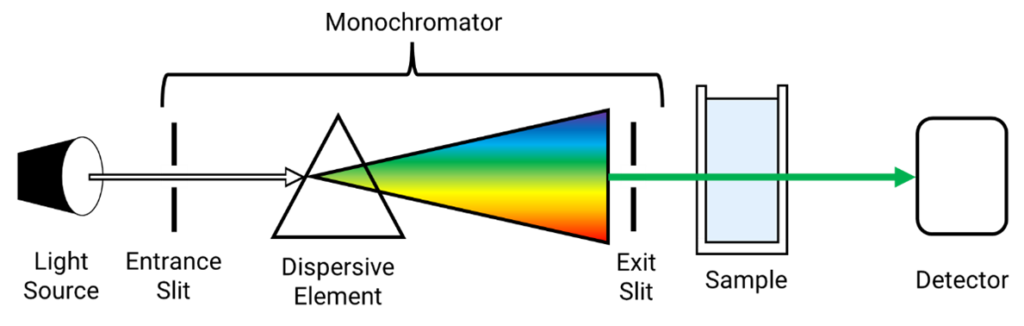
There are certain classes of drugs that may not exhibit significant absorption in the UV range or may not be suitable for analysis using UV spectroscopy. Here are some examples of drugs that generally cannot be effectively analyzed by UV spectroscopy:
- Drugs without significant UV absorption: Some drugs do not possess strong absorption in the UV region and thus cannot be reliably analyzed using UV spectroscopy. Examples of such drugs include many antibiotics like vancomycin, macrolides such as clarithromycin, and certain anticonvulsants like gabapentin.
- Drugs that absorb in other regions: Some drugs may absorb strongly in regions other than the UV range, making UV spectroscopy less suitable for their analysis. For instance, many colored compounds or drugs with chromophores outside the UV range cannot be effectively analyzed using UV spectroscopy. An example is chlorophyll, which absorbs strongly in the visible region.
- Highly complex drugs: Drugs that are structurally complex or have multiple functional groups may not exhibit well-defined absorption patterns in the UV range. These compounds often have overlapping absorption bands or exhibit broad spectra, making UV spectroscopy less effective for their analysis. Examples include complex natural products like certain alkaloids or polymeric drugs.
- Metal-containing drugs: Drugs that contain metal ions or metal complexes may not be suitable for analysis using UV spectroscopy due to the influence of metal-ligand interactions on their electronic transitions. These drugs often require other techniques, such as atomic absorption spectroscopy or inductively coupled plasma mass spectrometry (ICP-MS), for accurate analysis. Examples include cisplatin, a platinum-containing anticancer drug, or ferrous sulfate, an iron-containing supplement.
In cases where UV spectroscopy is not suitable or sufficient, other analytical techniques like HPLC, gas chromatography (GC), nuclear magnetic resonance (NMR) spectroscopy, or mass spectrometry (MS) are employed for the analysis and characterization of drugs. It’s important to consider the specific properties and requirements of each drug when selecting the appropriate analytical method.